
Recently, Associate Professor Wang Tao from our college has made significant progress in the research on aqueous lithium-ion battery electrolytes. This research project was jointly conducted with Professor Zhi Chunyi from the City University of Hong Kong, and the relevant results were published in the top materials journal Advanced Functional Materials as a research paper titled "Effective water confinement and dual electrolyte-electrode interfaces by zwitterionic oligomer for high-voltage aqueous lithium-ion batteries". Deng Yinyan, a doctoral student from our college, is the first author of this paper, while Associate Professor Wang Tao and Professor Fei Linfeng are co-corresponding authors. Nanchang University is the first corresponding institution. This research has received support from the National Natural Science Foundation of China and the Natural Science Foundation of Jiangxi Province.
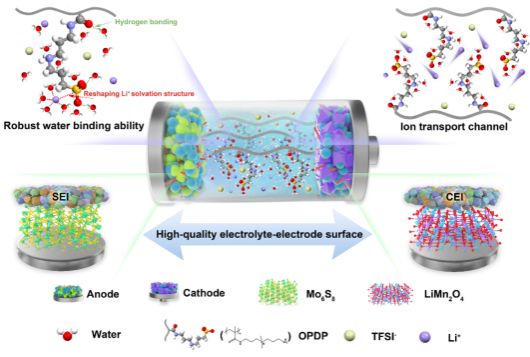
Lithium-ion secondary batteries have garnered widespread attention due to their remarkable characteristics, including long-cycle stability and high energy density. However, the toxicity and flammability of organic electrolytes pose significant safety hazards. In this context, replacing organic electrolytes with aqueous electrolytes emerges as an attractive solution, effectively mitigating safety and cost concerns. Nevertheless, the narrow electrochemical stability window of water (≈1.23V) severely constrains the selection of electrode materials for aqueous lithium-ion batteries. Additionally, the high reactivity of water leads to structural instability in electrode materials, resulting in a continuous decrease in reversible capacity and a decline in coulombic efficiency. Therefore, enhancing the electrochemical stability of aqueous lithium-ion battery electrolytes poses a significant challenge.
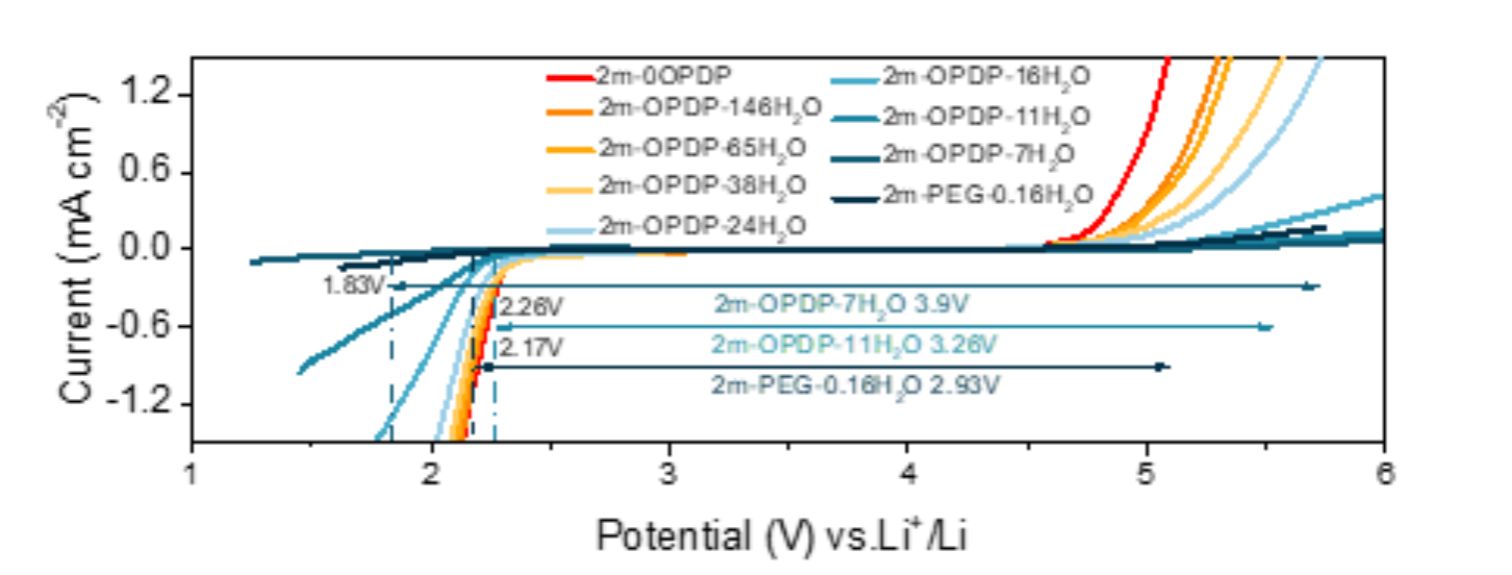
The research team obtained an aqueous electrolyte with a high electrochemical stability window by designing a low molecular weight zwitterionic oligomer as an additive. This oligomer exhibits excellent hydration capacity, significantly enhancing the hydrogen evolution and oxygen evolution potentials of the electrolyte. Simultaneously, the zwitterionic groups facilitate ion transport, resulting in high ionic conductivity of the electrolyte. Furthermore, with the assistance of this additive, effective formation of SEI and CEI protective layers on the electrode interface provides additional kinetic barriers for hydrogen evolution and oxygen evolution reactions. The full-cell based on this electrolyte demonstrates excellent cycling stability and rate performance. This research outcome offers a new approach for the development of high-performance and low-cost aqueous batteries.
Paper link:
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202416566

